Congenital Heart Disease
PROJECT 1: Prenatal cardiac load modulation to study the impact on chamber growth and maturation
|
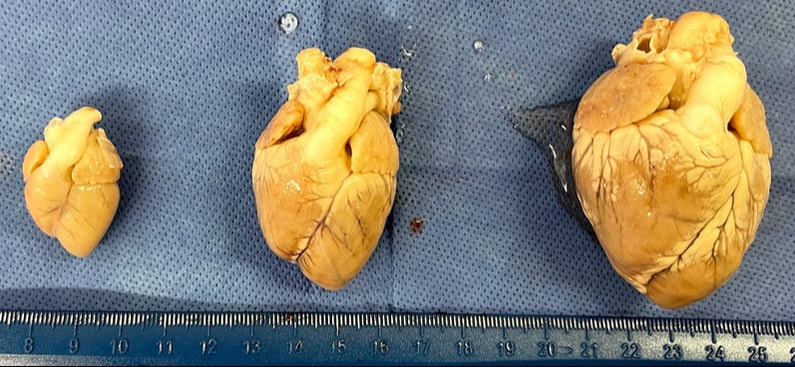
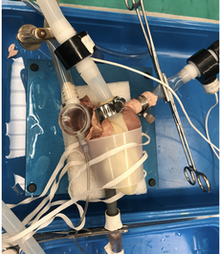
Pre-natal loading of the cardiac chambers can govern the post-natal size, function and structure of the cardiac chambers. Overloading or under loading the heart in gestation can have severe implication on the chambers, cardiomyocyte size and maturation. Prior work in chick embryos has demonstrated this link, but translation of this work to understanding human single ventricle defects is not yet established. In our lab we are trying to develop pre-clinical models that help us in understanding the link between cardiac chamber loading and its maturation in the prenatal period. Our long term goal is to understand if such models can yield a congenitally abnormal heart that is alike the patient, so that post-natal studies can be performed. This project is being performed in collaboration with Dr. Jonker's lab at OHSU.
|
PROJECT 2: Piggy back heart (PBH) for single ventricle patients palliated with a Fontan surgery
|
Completion of the stage III fontan circulation in children with single ventricle defects, leaves them with a venous circulation that lacks a pump to drive flow into the pulmonary system for oxygenation. This causes pooling of blood in the inferior vena cava, and significant rise in central venous pressure, which can be deleterious to liver function, gut function, lymphatic drainage, all of which have debilitating consequences .
In this project, we are developing a new circulatory pump for the venous circulation, to aid flow from the peripheral circulation into the pulmonary bed. The pump is being designed to be self-powered harvesting motion of the native heart, with a back-up power from a miniature linear actuator in the chest. |
PROJECT 3: Implantable devices to treat atrioventricular valve regurgitation in Fontan patients
Nearly 50% of the patients who received a Fontan surgery to palliate their single ventricle heart, develop clinically significant atrioventricular valve regurgitation. AVVR is debilitating for the child, as the energetics of the heart are abnormal, and the volume overload from the AVVR can lead to gradual failure of the single, viable chamber of the heart. As the child already underwent 3 surgeries, performing a 4th surgery to repair the patients AVVR is a risky procedure.
In this project, we are developing a novel implant that can potentially repair AVVR without the need for surgery. The implant is envisioned to be delivered into the beating heart onto the atrioventricular valve, using a catheter, via a fenestration from the Fontan graft into the right atrium. Our hope is that this cath procedure may relieve the patient's AVVR and increase their life well beyond what is possible today.
In this project, we are developing a novel implant that can potentially repair AVVR without the need for surgery. The implant is envisioned to be delivered into the beating heart onto the atrioventricular valve, using a catheter, via a fenestration from the Fontan graft into the right atrium. Our hope is that this cath procedure may relieve the patient's AVVR and increase their life well beyond what is possible today.